Eyeon Therapeutics has received a Notice of Allowance patent on a dry eye treatment based on a charged hydrophilic polymer developed by Particles Sciences
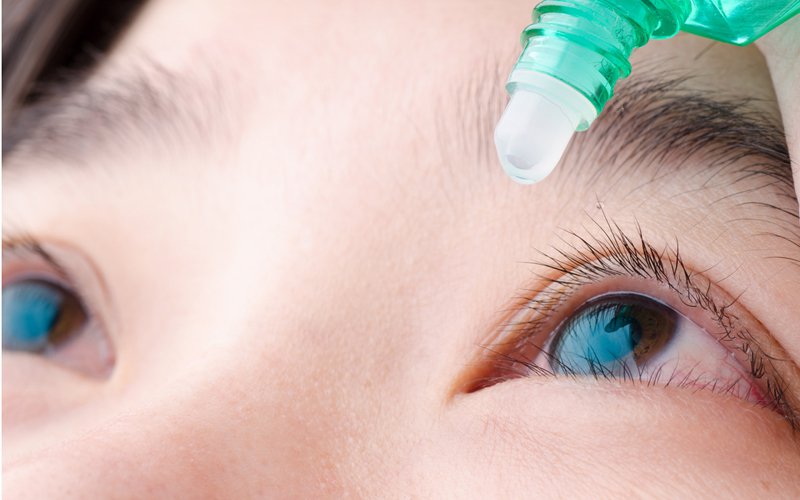
Particle Sciences, now a Lubrizol LifeSciences company, developed the polymer for Eyeon Therapeutics of Rochester which will develop the finished product.
According to Eyeon the product has been shown to be safe and effective in a small trial that was previously published.
Mark Mitchnick, CEO of Particle Sciences said: "This first set of claims around the polymer and its class will go a long way to moving this technology into the commercial phase.
“We have been using it in several development projects and we believe it has promise in many ocular formulations as well as in other areas.
“Helping Eyeon gain a proprietary position in this very important market is a great example of what Particle Sciences offers its partners."
David Kleinman, CEO of Eyeon Therapeutics, said: "Dry eye is a serious and growing problem for which there are few therapies.
“Our product offers a unique approach that is clinically validated and useful in a number of ocular applications.
“In the coming months we will be pursuing partnerships and expanded protection around this product and follow-ons."

